MegaTox is a collection of hundreds of curated and validated ADME/toxicology machine learning models addressing in vitro and in vivo datasets including: hepatotoxicity, cytotoxicity, mutagenicity, cardiotoxicity, drug-drug interactions, microsomal stability, Pregnane X receptor (PXR), drug-induced liver injury (DILI). We have an extensive number of models relevant to ecotoxicology as well as the acute toxicity “six-pack” and various models to predict LD50. We include datasets that we have curated ourselves and from our publications. This also includes models generated with large public datasets from ChEMBL, PubChem, Tox21, and EPA datasets.
MegaTox can assist companies meet their goals for sustainable chemistry by enabling the evaluation of materials produced or used. Predictions could also be used for regulatory assessments and filings.
Building on the work of Helman, Shah and Patlewicz describing generalized read across - GenRA (Comput Toxicol. 2018 ; 8: 34–50) and integrated in the CompTox Chemicals Dashboard, (Helman et al., ALTEX 36(3), 462-465) we have integrated a read-across tool in MegaTox. The following images illustrate this in action.

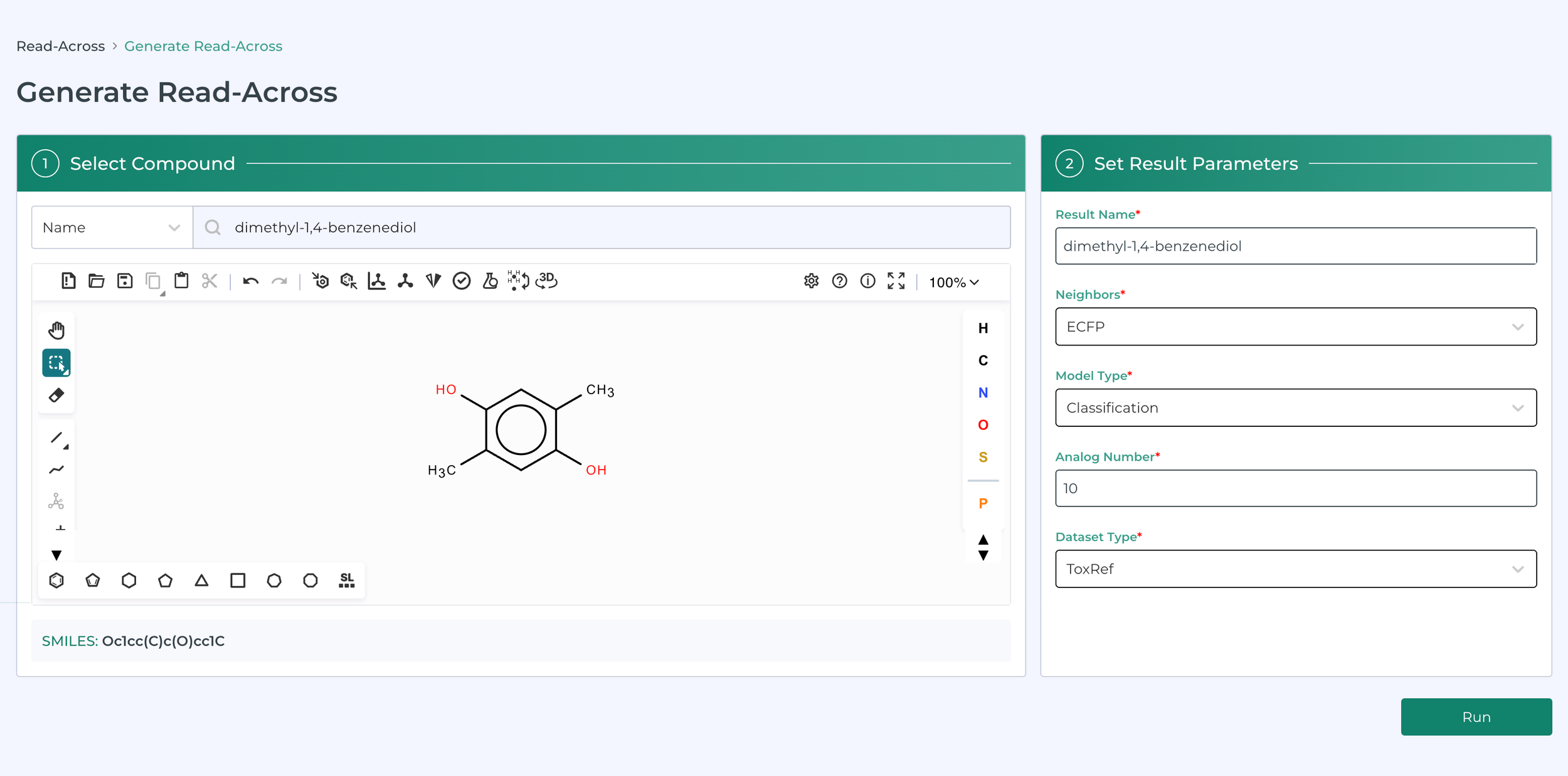
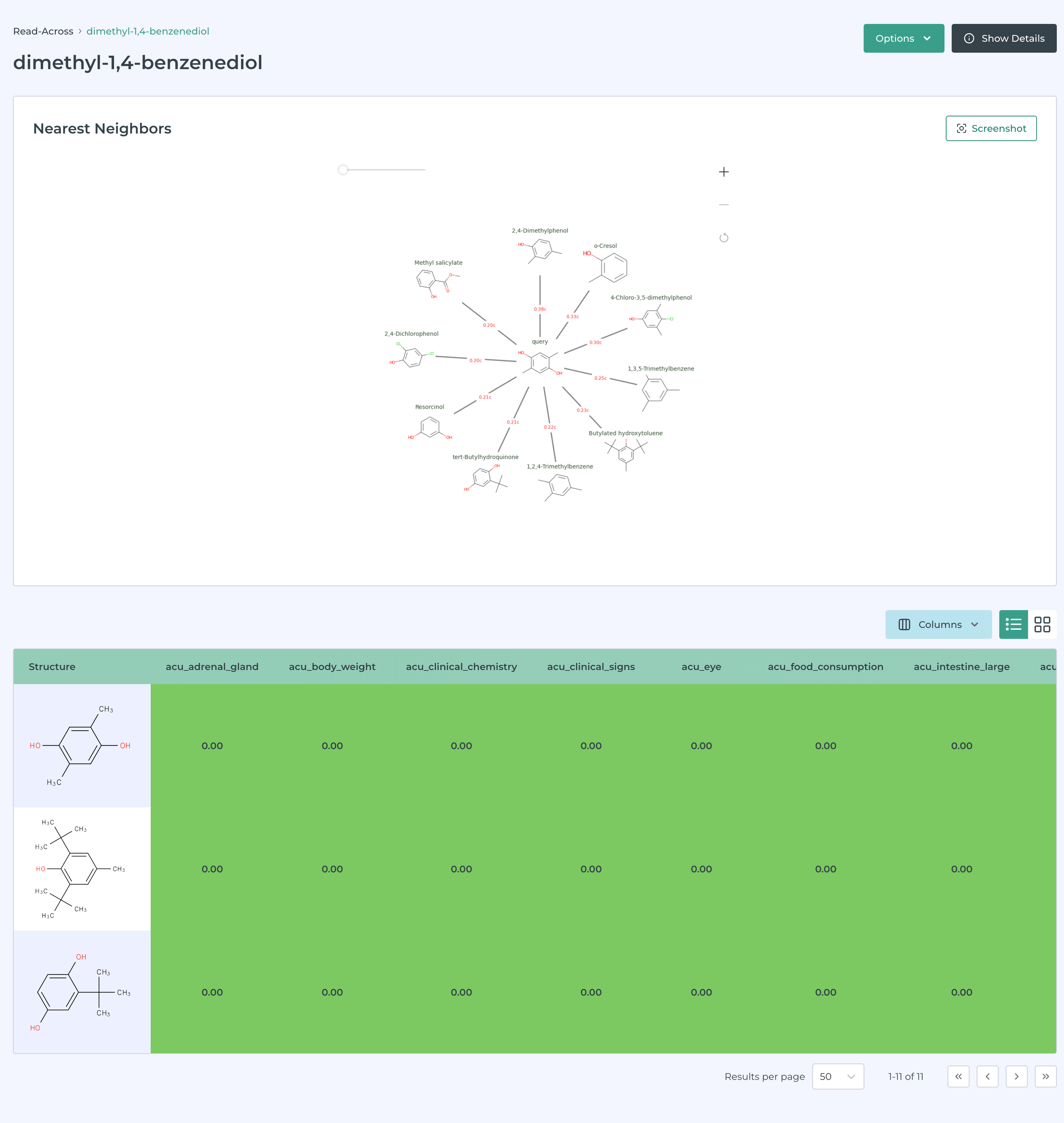
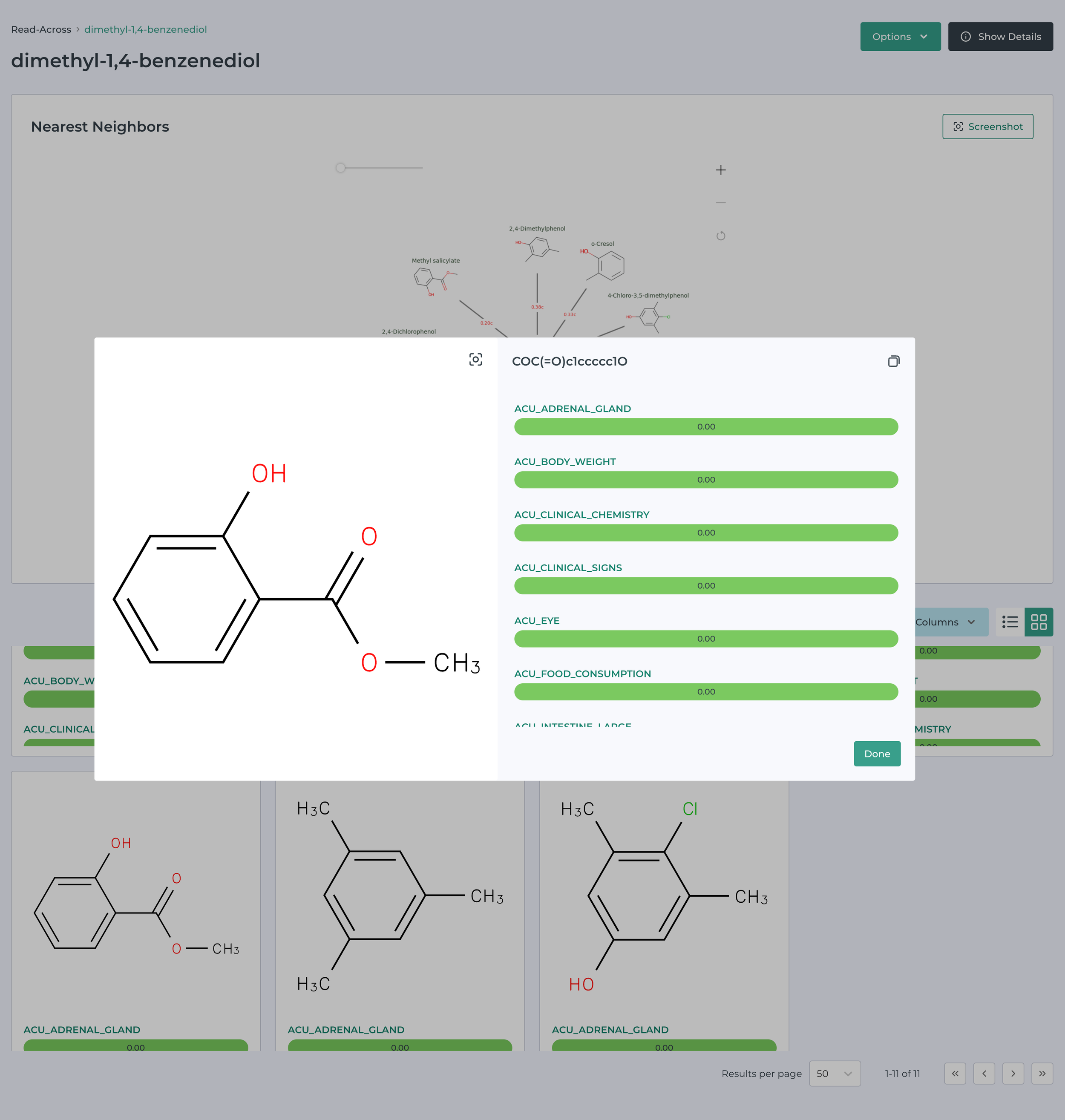
This software can be used to prioritize molecules or ingredients for pharmaceutical, consumer product, animal health and agrochemical companies.
Access
- We can use MegaTox in fee for service work for you.
- We can combine MegaTox and read across in a single package.
- We can provide an annual license for you to access this software on your own server.
- We provide maintenance and customization options.
